Get full access with a free account
Benefits of the Coloplast® Professional Educational platform
- Get full access to all educational content, events and resources
- Track your progress
- Share content with your colleagues
- Share supporting material with your patient
The complex and ever-changing healthcare landscape can lead to questions around reimbursement and coverage of intermittent catheters (IC). This page is a resource to better understand reimbursement and coverage for IC, including recent coding changes that may affect how these products are billed and reimbursed. Staying informed can be crucial for ensuring proper coverage and avoiding potential issues with claims.
For any questions, please reach out to our Reimbursement and Market Access team at USreimbursement@coloplast.com
What is new
Medicare Urological Supplies Local Coverage Determination (LCD) and Policy Article will go into effect January 1, 2026!
The LCD incorporates 3 new hydrophilic catheter HCPCS codes and expands access to closed catheter systems to all individuals with a spinal cord injury (any-level).
Medicare program outline1
Medicare is a federal insurance program that covers individuals who are 65 years of age or older, people under age 65 with certain disabilities and people of all ages with end-stage renal disease (permanent kidney failure requiring dialysis or a kidney transplant).
Medicare is an 80/20 plan, meaning Medicare covers 80% and the remaining 20% is the beneficiaries responsibility.
For any item to be covered by Medicare, it must:
- Be eligible for a defined Medicare benefit category,
- Be reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member, and
- Meet all other applicable Medicare statutory and regulatory requirements. Information provided in this policy article relates to determinations other than those based on Social Security Act §1862(a)(1)(A) provisions (i.e. “reasonable and necessary”).
Part A - Hospital Insurance
- Hospital Stays
- Skilled Nursing Facility
- Hospice
- Some Home Health
Part B - Medical Insurance
- Outpatient care
- Physical & Occupational Therapy
- Durable Medical Equipment (DME) - medical supplies included
- Some Home Health
Part C - Medicare Advantage
- Commercial Insurances offer Medicare Benefits
Part D - Prescription Drug Coverage
- Beneficiary pays a monthly premium
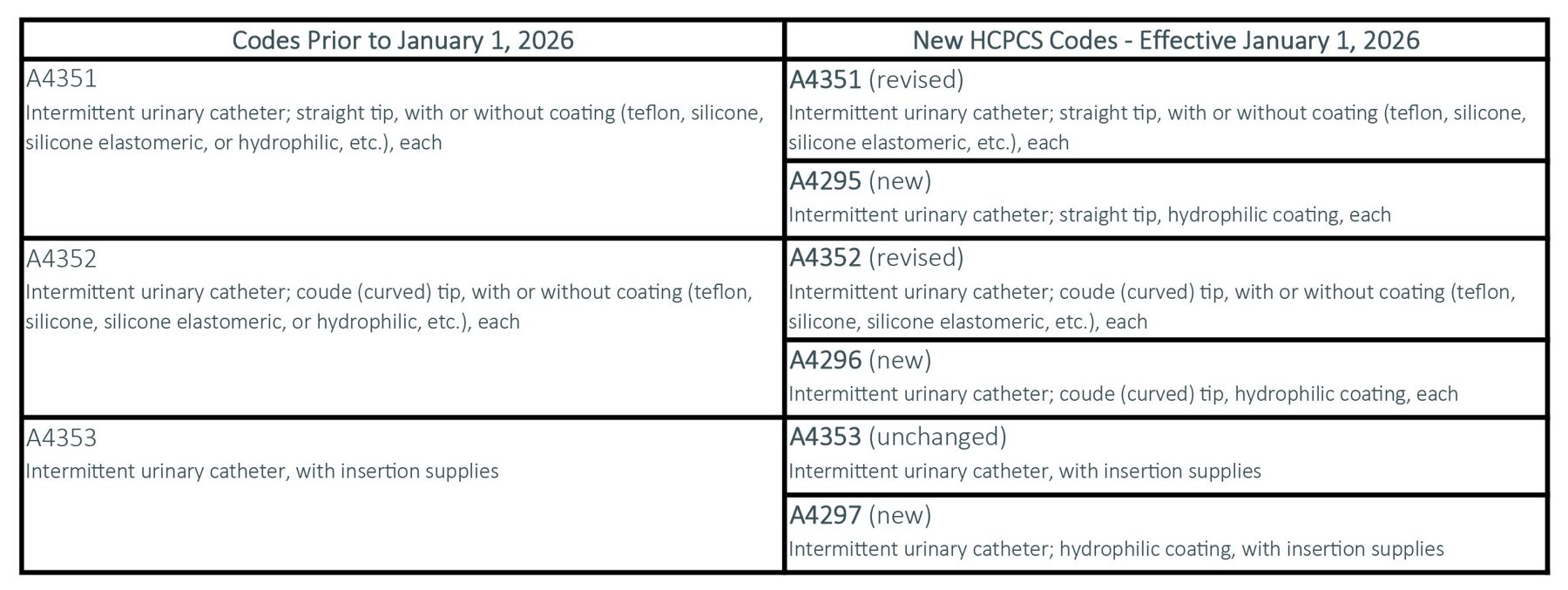
New HCPCS Codes for Intermittent Catheters
Background:
On August 16, 2024, the Centers for Medicare & Medicaid Services (CMS) announced changes to the HCPCS code set for intermittent catheters. This includes the modification of two existing codes and the creation of three new codes to specifically identify intermittent catheters with a hydrophilic coating. These changes acknowledge the clinical value of hydrophilic coatings and are intended to improve patient access and encourage continued innovation in urological care.
To support the successful adoption of new and revised HCPCS codes related to intermittent catheters, we encourage taking the following steps:
- Familiarize yourselves with the new HCPCS codes and updated code descriptions related to hydrophilic catheter technology: A4295, A4296, and A4297.
- Coordinate with your billing and coding teams to ensure accurate documentation and coding practices that reflect these updates.
- Advocate for appropriate reimbursement by working with your payer contacts to ensure coverage supports patient access to hydrophilic coated catheters.
The Pricing, Data Analysis and Coding (PDAC) contractor created a Hydrophilic Intermittent Urinary Catheter Crosswalk PDAC - Hydrophilic Intermittent Urinary Catheter Crosswalk
Additional information and resources on the New HCPCS Codes for Intermittent Catheters can be found on the American Association for Homecare website
CMS published prescription guidelines for hydrophilic catheters on July 17th that can be found here: Documentation Requirements for New Urological Supplies Codes
The following chart provides an overview on the HCPCS code changes:
Webinar Topic: New Intermittent Catheter HCPCS Code Changes
On January 1, 2026, the Centers for Medicare and Medicaid Services (CMS) will make HCPCS code changes for intermittent catheters. The changes include the modification of two existing codes and the addition of three new codes to differentiate catheters with a hydrophilic coating.
![]() Date: November 4th, 2025 at 12pm EST
Date: November 4th, 2025 at 12pm EST
Speakers: Noel Neil & Anna Markiewicz
Hosted by: Coloplast & AcuServe
Webinar Topic: Preparing for Upcoming Intermittent Catheter HCPCS Code Changes
Join Noel Neil, VP of Auditing and Corporate Compliance at AcuServe, and Anna Markiewicz, Sr. Manager of Payor Relations at Coloplast, as they break down the new coding structure and discuss opportunities for DME providers.
![]() Date: July 15, 2025 at 12pm EST
Date: July 15, 2025 at 12pm EST
Speakers: Noel Neil & Anna Markiewicz
Hosted by: Coloplast & AcuServe
Medicare coverage for intermittent catheters 2,3
- Intermittent catheters (IC) are covered for Medicare beneficiaries who have a permanent impairment of urination. This is generally defined as a condition of long and indefinite duration.
- Medicare covers three types of intermittent catheters.
- Exact quantity of IC per month is determined by the clinician and is based on what is reasonable & necessary for each patients' individual need
- Click here for the most up-to-date Urological Medicare Policy and Urological Medicare Article
What products are considered A4353/A4297?
A4353/A4297 is a kit, which includes a catheter and all supplies necessary for a single, sterile insertion (see below). Code A4353/A4297 may be used if any of the following 1, 2 or 3 is supplied:
- A single sterile package containing both an intermittent urinary catheter and all necessary insertion/collection supplies;
- A sterile intermittent urinary catheter plus a separately-packaged sterile kit containing all necessary insertion/collection supplies;
- A sterile "no-touch" type of catheter system
The product described in #3 is a single-catheter system that is functionally equivalent to a complete sterile insertion kit (A4353/A4297) containing a catheter and the additional components as described in the previous paragraph. In order to be coded as A4353/A4297, a "no-touch" type of catheter system must be a sterile, all-inclusive, self-contained system capable of accomplishing intermittent catheterization with sterile technique without the use of additional supplies such as gloves, lubricant, collection chamber, etc.
Medicare documentation for intermittent catheters 2,3,4
General Medicare documentation list
Prescription:
- Patients’ information (name, date of birth)
- Type of IC prescribed (general description, a HCPCS code, a HCPCS code narrative, or a brand name/model number)
- Quantity of IC (specific number)
- Prescribing clinicians’ signature
- Clinician name or National Provider Identifier (NPI)
- Order date
Medical Record: *
- Documentation of permanent urinary incontinence or permanent urinary retention
- Medical justification for a type of intermittent catheter that is being prescribed
- Must match the prescription (frequency of IC, quantity of IC, type of IC)
Click below for the intermittent catheters documentation checklists from Noridian and CGS:
Noridian Documentation for Urological Supplies
CGS Documentation for Urological Supplies: Intermittent Cathers
Medicare documentation required by intermittent catheter type*
Hydrophilic Intermittent Catheters
A4295 - Intermittent urinary catheter; straight tip, hydrophilic coating, each
- Everything in the general Medicare documentation list
A4296 - Intermittent urinary catheter; coude (curved) tip, hydrophilic coating, each
- Everything in the general Medicare documentation list
- Documentation indicating that patient has tried and is unable to pass a straight tip catheter
- Documented medical need for a coude catheter
A4297 - Intermittent urinary catheter; hydrophilic coating, with insertion supplies
- Everything in the general Medicare documentation list
- Patient meets one of 4 criteria:
- Patient resides in a nursing facility
- Patient is immunosuppressed:
- on a regimen of immunosuppressive drugs post-transplant
- on cancer chemotherapy
- has AIDS
- has a drug-induced state such as chronic oral corticosteroid use
- has a diagnosis of spinal cord injury at any level
- Patient has documented vesico-ureteral reflux
- Patient has had 2 documented urinary tract infections (UTI) while on a straight or coude tip IC within 12 months
Required documentation for UTIs:
- Urine culture showing greater than 10,000 bacteria for each UTI
- One additional symptom:
- Fever
- Systemic leukocytosis
- Change in urinary urgency, frequency, or incontinence
- Appearance of new or increase in autonomic dysreflexia (sweating, bradycardia, blood pressure elevation)
- Physical signs of prostatitis, epididymitis, orchitis
- Increased muscle spasms
- Pyuria (greater than 5 white blood cells [WBCs] per high-powered field)
Non-Hydrophilic Intermittent Catheters
A4351 - Intermittent urinary catheter; straight tip, with or without coating (teflon, silicone, or silicone elastomeric, etc.), each
- Everything in the general Medicare documentation list
A4352 - Intermittent urinary catheter; coude (curved) tip, with or without coating (teflon, silicone, or silicone elastomeric, etc.), each
- Everything in the general Medicare documentation list
- Documentation indicating that patient has tried and is unable to pass a straight tip catheter
- Documented medical need for a coude catheter
A4353 - Intermittent catheter, with insertion supplies
- Everything in the general Medicare documentation list
- Patient meets one of 4 criteria:
- Patient resides in a nursing facility
- Patient is immunosuppressed:
- on a regimen of immunosuppressive drugs post-transplant
- on cancer chemotherapy
- has AIDS
- has a drug-induced state such as chronic oral corticosteroid use
- has a diagnosis of spinal cord injury at any level
- Patient has documented vesico-ureteral reflux
- Patient has had 2 documented urinary tract infections (UTI) while on a straight or coude tip IC within 12 months
Required documentation for UTIs:
- Urine culture showing greater than 10,000 bacteria for each UTI
- One additional symptom:
- Fever
- Systemic leukocytosis
- Change in urinary urgency, frequency, or incontinence
- Appearance of new or increase in autonomic dysreflexia (sweating, bradycardia, blood pressure elevation)
- Physical signs of prostatitis, epididymitis, orchitis
- Increased muscle spasms
- Pyuria (greater than 5 white blood cells [WBCs] per high-powered field)
Effective January 1, 2026 - New Local Coverage Determination (LCD) for urological supplies!
Summary of changes:
1. Addition of three new hydrophilic catheter HCPCS codes:
- A4295 – straight tip hydrophilic
- A4296 – coude tip hydrophilic
- A4297 – closed system hydrophilic
2. Hydrophilic catheter definition:
- A hydrophilic catheter is a type of (not same as) pre-lubricated catheter having a polymer coating that
binds water to the catheter to make it slippery. The hydrophilic coating on a catheter is intrinsic to the
catheter product (i.e., you cannot wipe the coating off). Because hydrophilic catheters are self-lubricating, the
use of a separate lubrication product is not necessary.
3. Expansion of access to closed intermittent catheters (A4297/A4353) for individuals with a spinal cord injury
- Spinal cord injury at any level is now included under the immunosuppression qualifying (criteria #2)
- A diagnosis of a spinal cord injury in the medical record is needed to qualify for a closed system catheter
(A4297/A4353)
Medicare resources
- Medicare Urological Supplies Policy LCD - Urological Supplies (L33803) (cms.gov)
- Medicare Urological Supplies Article Article - Urological Supplies - Policy Article (A52521) (cms.gov)
- 2025 Medicare DME fee schedule DMEPOS Fee Schedule | CMS
- Documentation checklist for intermittent catheters: Noridian Documentation Checklist for Urological Supplies and CGS Documentation Checklist for Urological Supplies: Intermittent Catheters


Webinar: Preparing for upcoming intermittent catheter HCPCS code changes
Webinar: Navigating through Medicare qualifications for closed system intermittent catheters